The medical industry has exploded in recent decades with new technologies, new materials, and new capabilities, and we have benefited in both quality and duration of life. One of these many advances entails the intersection of medical devices and machined parts. What historically was a laborious hand- and molding process can now be done quickly, effectively, and cost-efficiently in several materials for applications prior generations never thought feasible.
However, these new devices, which include surgical implements, medical implements, and medical devices, require new maintenance protocols that prior manufactured parts did not. 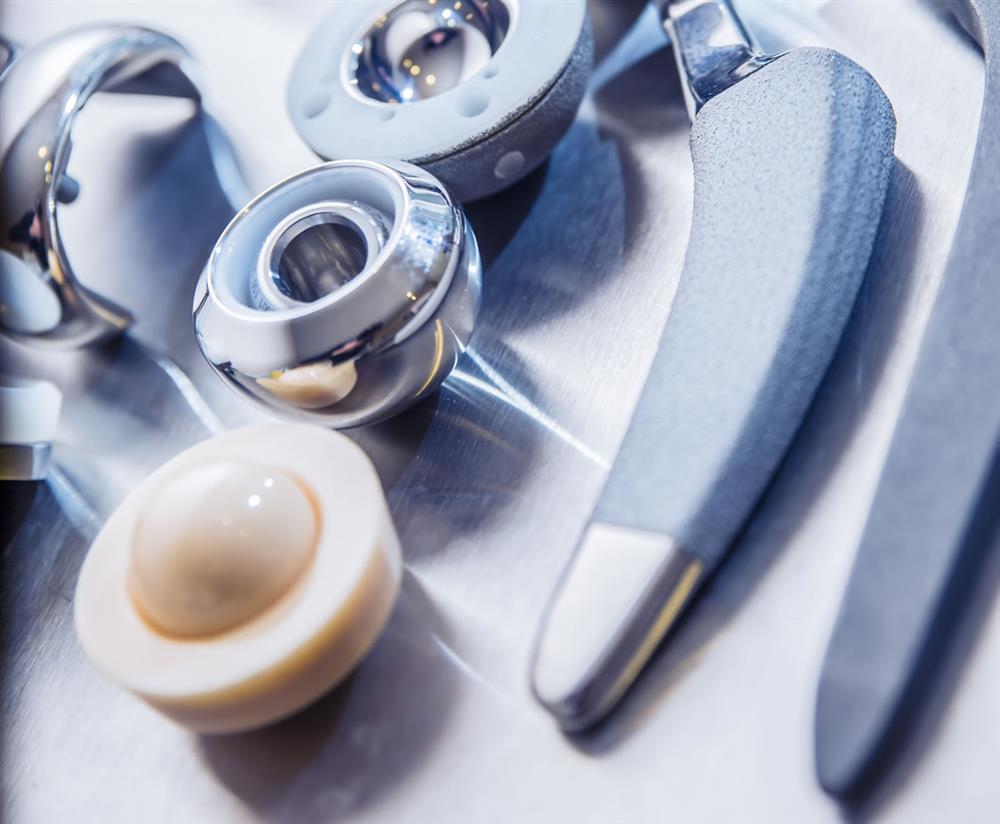
Common medical implants that require machining and cleaning.
Let’s look at the current CNC machining process for medical implants, the cleaning they demand, the options that exist, and the circumstances to have led us to this change in procedure.
Overview of CNC Machining of Medical Implants
CNC machining is a profoundly enhanced process over conventional manufacturing, in both speed and precision. Machined products can meet tight tolerance requirements as well as performance standards. The devices can be directly formed (machined), but these processes can also generate the precise molds used in secondary steps for molded devices. In either approach, the resultant product is ideal for implants, medical equipment and surgical instruments.
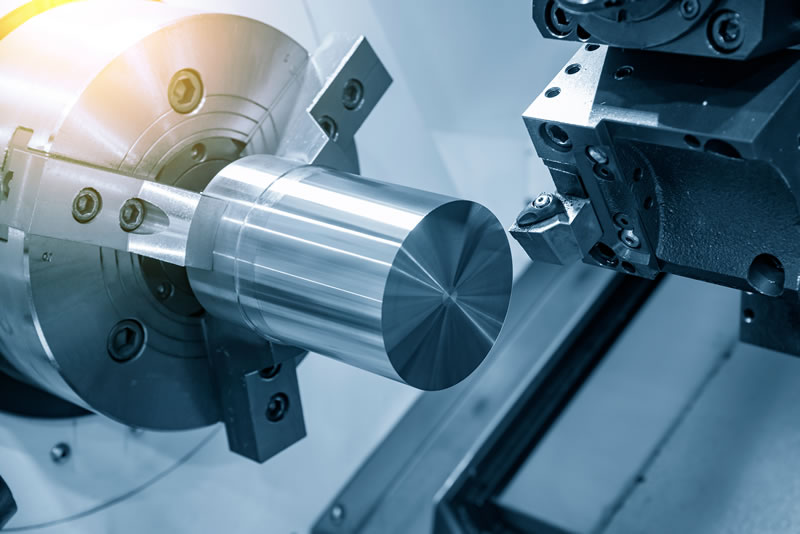
CNC machining of a metal rod.
But they are not without the need for final preparation after manufacture. Residues from the process, which may include release agents, machining media, chips, or dust must be removed. However, these must be removed in a way that is biocompatible with the resultant application.
The cleaning industry offers various cost-effective and ecologically-safe methods to achieve these goals. The cleaning methods include wet chemical cleaning processes, plasma processes, cleaning with carbon dioxide, as well as combinations of the three or other steps.
Different types of applications
CNC machined products can be formed or in any number of materials, each suited for different applications and requiring different cleaning treatments.
|
Material |
Overview |
Medical Applications |
|
Aluminum |
A strong, lightweight material with good corrosion-resistant properties. Can be anodized for mechanical and corrosion resistance properties |
Not ideal in direct contact with human body. Excellent for support equipment. |
|
Stainless Steel |
Biocompatible, durable, strong and corrosion resistant. Can be polished for easy cleaning |
Orthopedics like joint replacement, and surgical devices |
|
Copper |
Antibacterial and antiviral |
Frequently touched surfaces (switches) and dental implants |
|
Titanium |
Often substituted for stainless steel due to strength, durability and biocompatibility. |
Bone replacement, prosthetics |
|
Magnesium |
Excellent strength to weight ratio. Biosafe. Safely biodegrades |
Bone graft replacement and heart stents |
|
Delrin |
High-strength, rigid, and dimensionally stable thermoplastic. Chemically resistant, low coefficient of friction |
Orthopedic implants, surgical instruments, inhalers, simple components |
|
Acrylic |
Resistance to impact, optical clarity. |
Contact lenses, implants, dental prosthetics, microscopes and shields |
|
Polycarbonate (PC) |
High strength, impact resistant and optical clarity. |
Tubing and connectors for devices, goggles, masks, handles and housing. |
|
PEEK |
Strength and exceptional high performance properties. Highly chemical resistant. |
Parts exposed to harsh chemicals. Ventilator tubes, clamps, prosthetics, Xray machines. |
|
PTFE (Teflon) |
Medical-grade thermoplastic with high heat resistance and good dimensional stability. Reliable in repeated steam sterilizations. |
Catheters, implantable devices, prosthetics, wound dressings. |
|
Polypropylene (PP) |
High chemical resistance, good dimensional stability, high strength, wear resistance. |
Test tubes, pipettes, medical packaging, inhalers, disposable medical devices. |
|
UHMWPE |
Biocompatibility, high strength, wear resistance. |
Implants, shaves, catheters, splints. |
|
Nylon |
Versatile engineering grade. Tough and strong. |
Sutures, catheters, forceps, clamps, implants, dental devices. |
With all these applications, it is important to engineer those that will allow easy maintenance. This will ensure that the parts will continue to function properly and safely for the duration, paying dividends in saving money in both the maintenance itself and in the life cycle of the device.
CNC machining of dental implants.
Using durable and easily cleaned materials is one way to achieve this, but also the installation of any monitoring devices into the CNC manufacturing process to identify any anomalous issues.
Overview of Cleaning Options for Implants
There are number of demonstrated processes that are effective for cleaning medical implants. Selecting the appropriate one depends on the material, on the biochemical requirements, on the manufacturing process completed, and the intended use. In addition, the facility and staff employed in the cleaning, storage, and use of the material as well as any patient use must be factored. Costs, both in terms of raw materials and in use-costs, must be considered, in addition to local regulations and changing circumstances from the environmental review process.Following is an overview of the common and new processes developed.
Wet Cleaning
The traditional bulk cleaning process is the Economic Batch process in Wet cleaning. Bulk goods baskets are laid out to contain the medical parts that require cleaning. Different applications benefit from different rack configurations, as well as the chemistry used, duration and other malleable process steps. Racks of round wire have become the preferred configuration for effectiveness. Various chemical agents are employed, largely reliant on the materials. Although common in most applications, there are newer processes detailed below that may be more suitable than wet cleaning.
Supercritical Carbon Dioxide
Supercritical Carbon Dioxide is used in aggregation state (between liquid and gaseous state) when utilized in cleaning machined parts. In this state, it has minimal viscosity and surface tension which are important considerations in mass transport—which is how it can remove stubborn contamination (oils, greases) from even the smallest pores.
Performed between 20–40° C (68–104°F), supercritical carbon dioxide can achieve good results in porous structures, and can enhance needed biological parameters more so than conventional processes through lower cytotoxicity. Uses for CO2 cleaning in medical engineering include intermediate and final cleaning of implants, instruments, and components from metals and plastics.
CO2 Snow
CO2 is a cleaning agent much like the Supercritical Carbon dioxide but as it is frozen, it is a dry product that results in no residue. Liquid carbon dioxide is expanded through a nozzle and accelerated to ultrasonic speeds with compressed air. The CO2 snow jet gently removes film-like contamination and particulates. It is suited to nearly all materials, including metal, plastic, glass, or ceramics.
Since it is a jet stream, it can be focused to restrict the functional jet to those areas needing that level of cleaning. It can be used in inline processes, with minimal space requirements, making it ideal for integration in the manufacturing process which ensures sterility of the final product as there is no transit prior to the final sealing.
Plasma
Plasma is a mixture of atoms, molecules, ions and free electrons in gaseous form. Medical devices from most materials can be treated in batch or in individual cleaning processes. Different plasma gasses can be used, simultaneously cleaning and activating the surface. This dual function derives from both the physical and chemical reactions. The atoms bombard the surface, like a miniature sand-jet in the nano range which removes organic contamination such as oil or grease. Meanwhile, free ions and electrons are deposited and chemically bond with the surface—which adjusts the surface tension to ideally engage in bonding, coating, or printing steps.
Common applications for plasma cleaning include final cleaning of stents and implants, guide wires pre-coating, silicone implants, catheters, syringes and to increase the surface energy of diagnostic instruments.
Vapor Degreasing Advantages
Even with these advanced cleaning methods, for most applications the simplest and most traditional is the most cost effective for many uses. Vapor degreasing remains the primary mode of cleaning for all but a select few applications or materials. Through condensing solvent vapors, the object is cleaned. No water or scrubbing is required. This cleans many materials during manufacturing process, including plastic, glass, metal, gold and ceramic.
Simple vapor degreasing contains two sump tanks, basket, and cooling coils. The first sump boils the solvent and the second sump collects the solvent distillate.
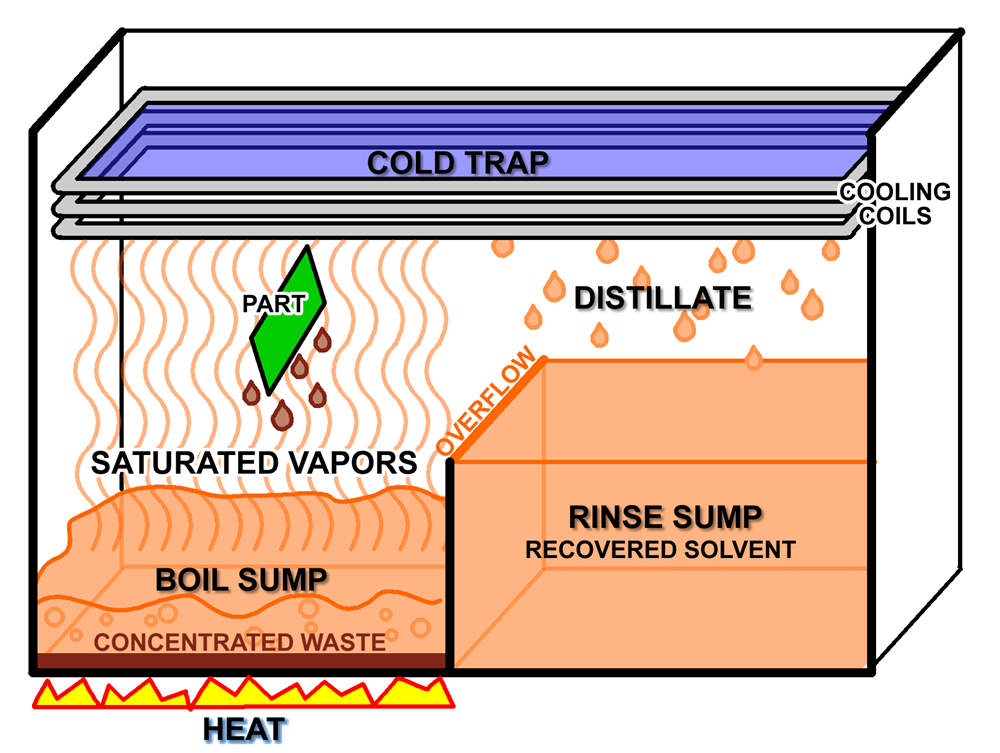
More complicated systems may include an immersion tank with secondary ultrasonic step for agitation cleaning.
Vapor degreasing is common in many industries requiring precise cleaning, including automotive, aviation and aerospace, jewelry, electronic assembly, and in medical devices. It offers superior cleaning, can be repeated, and does not rely on operator technique, minimizing human error.
Vaporized solvents avoid cross-contamination, and there is no need for a secondary drying process as the solvents dissipate quickly.
The solvents used in vapor degreasing are generally expensive, but with a closed loop process they are continuously recycled and so the cost per item is rather low in quantity production. Compared to processes used with aqueous cleaners, vapor degreasing doesn’t require heating water, rinsing or drying, or treating wastewater.
Explanation of NOVEC Announcement from 3M and Techspray Options
There are a number of solvents used in vapor degreasing. But one common solvent that is used in potentially flammable scenarios will be removed from production. NOVEC, the brand line made by 3M, uses PFAS as a critical ingredient in flame extinguishing applications and in flame retardant solvents. PFAS (Polyfluoroalkyl (and perfluoroalkyl) Substances) have been undergoing increased scrutiny by environmental regulators. Proactively, in December 2022, 3M announced their plans to phase out PFAS-based product lines by the end of 2025.
“3M today announced it plans to exit per- and polyfluoroalkyl substance (PFAS) manufacturing and work to discontinue use of PFAS in its product portfolio by the end of 2025. This portfolio decision is based on careful consideration and a thorough evaluation of the external landscape, including multiple factors such as accelerating regulatory trends focused on reducing or eliminating the presence of PFAS in the environment and changing stakeholder expectations.”1
PFAS-based products have been found to have adverse implications for human health, and their use over the decades have led to water table contamination. These chemical components break down very slowly, and PFAS has been found in most human populations and in substantive parts of the food cycle and plants. To stem this, increasing regulations are being considered and manufacturers, such as 3M, are pivoting away from their use wherever feasible.
Given the scale, 3M’s announcement has profound implications for many manufacturers that have relied on NOVEC products. When it comes to solvents, there are many options viable for most situations that can exclude PFAS. But for those scenarios required flame retardant additives, PFAS are as yet unavoidable.
The nonflammable solvents outside PFAS are risky in other ways; most have been found to have direct and indirect negative effects on people or on the environment, and have restrictions on their use or have been outright banned in many countries.
Alternatives are being developed to address these gaps. At Techspray, our focus has always been on high-precision cleaning chemistries, including direct chemical replacements for NOVEC and other proprietary formulas.
Techspray has a large variety of solvents intended for vapor degreasing under the brand names PWR-4™ and Precision-V™. For replacing 3M Novec solvents, we have some direct chemical crosses and options that fit the same criteria, but at a higher cleaning performance.
These products are engineered to be less toxic than many other solvents commonly used in vapor-degreasers: e.g., TCE (Trichloroethylene, CAS #79-01-6), nPB (n-Propyl Bromide, CAS #106-94-5), and Perc (Perchloroethylene, CAS #127-18-4).
Techspray’s TechLab offers state-of-the-art cleaning, coating and analytical services to help customers qualify new products and optimize their processes. Cleaning equipment includes inline, batch, ultrasonic, and vapor degreasing systems. This equipment allows us to better duplicate your production environment for process optimization and troubleshooting.
Contact Techspray at 678-819-1408 or info@itwcce.com for free TechLab qualification testing. We are available to help qualify new cleaning processes, evaluate current processes, or troubleshoot contamination issues.
References
[1] 3M Announcement Letter, Dec. 20, 2022, David N. Schneider, VP Chemicals and Semiconductor, 3M Company, Electronics Materials Solution Division.
[2] Chemtronics. https://www.chemtronics.com/is-this-the-end-of-the-forever-chemicals-era
[3] Chemtronics. https://www.chemtronics.com/Novec-and-vertrel-solvent-replacement-cleaning-options-for-medical-instrument-and-implantable-devices
[4] Clean Water Fund. “PFAS: The Forever Chemicals.” Clean Water Action MA Fact Sheet, Apr. 2020, www.cleanwateraction.org/sites/default/files/MA_FactSheet _PFAS_04.14.20a.pdf.
[5] Modern Machine Shop. https://www.mmsonline.com/articles/cleanliness-in-medical-engineering
[6] Rapid Direct. https://www.rapiddirect.com/knowledge-base/cnc-machining-applications-in-medical-industry/
[7] Techspray. https://www.techspray.com/3m-announcement-of-Novec-phase-out-what-does-it-mean
[8] Techspray. https://www.techspray.com/vapor-degreasing-the-quick-guide